Welcome to our blog! Today, we are excited to share a groundbreaking development in the field of Alzheimer’s treatment. A new drug called Lecanemab is showing remarkable promise in slowing cognitive decline by targeting brain plaque associated with the disease.
Recent advancements in medicine are offering hope to individuals like Rolfe Johnson, who sought help for memory issues at the age of 75. With the guidance of neurologist Dr. Paul Schulz from UTHealth, Rolfe became the first patient in Texas to participate in a clinical trial for Lecanemab.
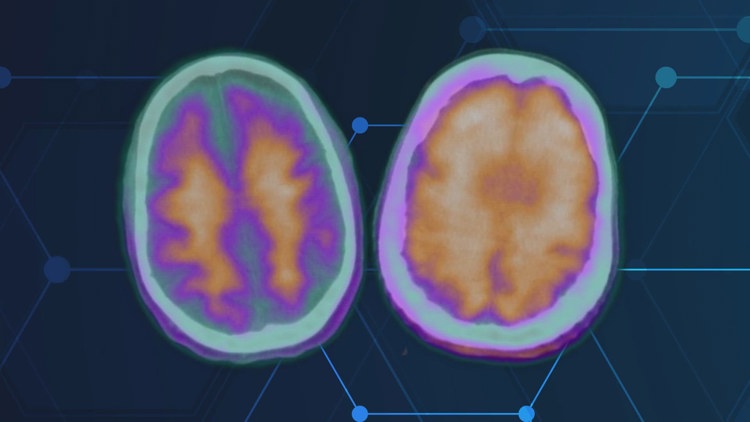
Lecanemab is a new class of Alzheimer’s drugs that work by using antibodies to target and eliminate amyloid plaques in the brain. Over time, this treatment can help reduce mental decline and even lead to cognitive improvement in some cases.
The FDA has approved Lecanemab, manufactured by Eisai, and it is now covered by insurance. Patients receive hour-long infusions every two weeks and undergo regular MRIs to monitor their brain health. Early intervention is crucial, as delaying treatment can impact the effectiveness of the medication.
For individuals like Rolfe and Carol Johnson, early access to this innovative treatment has made a significant difference in their quality of life. Despite some short-term memory issues, they continue to enjoy an active lifestyle and cherish their time together after 63 years of marriage.
If you or a loved one are interested in participating in a clinical trial for Alzheimer’s drugs, consider reaching out to UTHealth Houston for more information. Remember, early detection and intervention can make a world of difference in managing the impact of Alzheimer’s disease.
Thank you for joining us on this journey of discovery and hope in the fight against Alzheimer’s. Stay tuned for more updates and inspiring stories in the world of healthcare.

